Target RWE at #TLM2024
Learn about our activity at The Liver Meeting & our real-world evidence solutions
Explore Our Liver Disease Research
Target RWE attended and sponsored AASLD's 2024 The Liver Meeting event in San Diego where we presented new data on Metabolic Dysfunction–Associated Steatohepatitis (MASH) topics including racial and ethnic disparities, patient characteristics, disease progression, patient-reported outcomes, and GLP-1 receptor agonists. We additionally shared new primary biliary cholangitis (PBC) data related to complication-free survival. Learn more about our activity at the conference and how we have contributed to the ongoing advancements in patient care!
Proven Leadership in Liver Disease

“I’m thrilled to be attending The Liver Meeting this year in San Diego. It’s been incredible to witness the growth and progress of AASLD both during and after my presidency. Since my first attendance, I’ve been inspired by the dedication and innovation displayed each year at The Liver Meeting. I look forward to seeing the latest advancements in research and patient care in the field of Hepatology.” Michael W. Fried, MD, FAASLD Co-Founder, Chief Medical Officer & Former AASLD President
Notable Accomplishments:
- Founder and Co-principal investigator of the HCV-TARGET network – The highly successful collaborative framework for real world evidence generation
- Awarded the FDA Excellence in Regulatory Science Award for contributions to optimizing management for Hepatitis C
- Principal investigator of several NIH cooperative clinical trials
Strategic Partnership with AASLD
Target RWE's proud to collaborate with the American Association for the Study of Liver Diseases (AASLD) and have worked to successfully enroll over 600,000 patients in the TARGET-Liver Disease (LD) registry.
Our partnership facilitates the study of the natural history of disease and the evaluation of patient characteristics, disease management, treatments, and clinical outcomes among many subpopulations of patients with chronic liver disease, including MASLD, HBV, MASH, HCC, PBC, and PSC. The extensive data set supports AASLD's CQC initiative of a Learning Health Network to improve the quality, safety, and value of care for patients living with chronic liver disease and cirrhosis.

Our Research at The Liver Meeting 2024
#TLM2024 Presentations Progressing MASLD Research
Stability of the NASH-CHECK Patient-Reported Outcome Measure Over Time in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease and Stable Clinical Status
Co-author and co-chair of the TARGET-NASH steering committee, Arun J. Sanyal, MBBS, MD, presented a new Target RWE study analysis at The Liver Meeting 2024. This study evaluated the stability of NASH-CHECK® PROM scores at two time points in patients with MASLD whose clinical status remained unchanged between the first and second administration of the NASH-CHECK® PROM.
Use of GLP-1 Receptor Agonists in Patients with MASLD in a Real-World Setting Is Associated with Slower Disease Progression and Lower All-Cause Mortality
Member of the TARGET-NASH steering committee, A. Sidney Barritt, IV, MD, MSCR, presented a new Target RWE study at The Liver Meeting 2024 titled “Use of GLP-1 Receptor Agonists in Patients with MASLD in a Real-World Setting Is Associated with Slower Disease Progression and Lower All-Cause Mortality.”
Leveraging data from Target RWE’s metabolic dysfunction–associated steatohepatitis (MASH) patient cohort, the study highlights the potential of GLP-1 RAs to slow disease progression and reduce mortality among patients with MASLD.
A Prospective Assessment of the Impact of Decompensation of Cirrhosis on Patient-Reported Outcomes in Metabolic Dysfunction-Associated Steatotic Liver Disease
Co-author and co-chair of the TARGET-NASH steering committee, Arun J. Sanyal, MBBS, MD, additionally presented new research at the Liver Meeting 2024 conference on the NASH-CHECK patient-reported outcome measure.
The study addressed the impact of decompensated cirrhosis on MASLD-specific patient-reported outcome measures by comparing patients with MASH and compensated or decompensated cirrhosis in a real-world setting.
TARGET-LD: A Comprehensive Registry for Liver Disease Research in the Real World
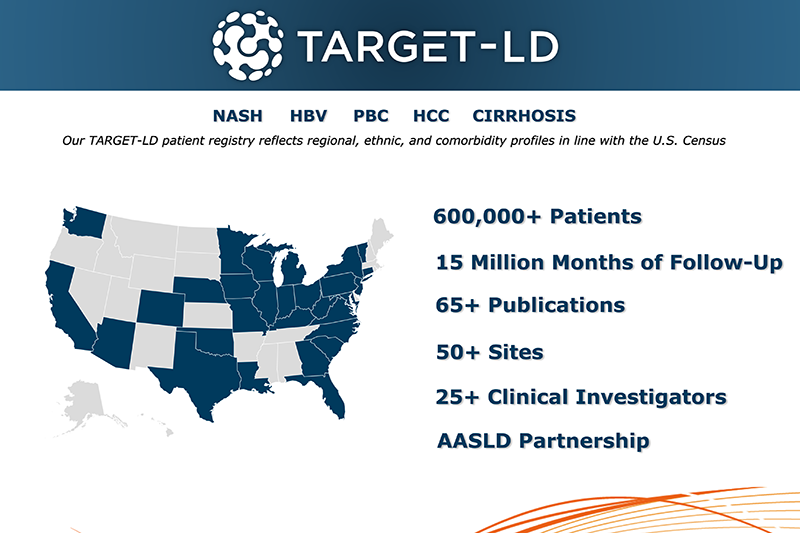
TARGET-LD is the world's largest liver disease registry, encompassing over 600,000 patients and capturing a diverse range of patient profiles. With its extensive patient population, diverse data collection, and strong partnerships, including AASLD, TARGET-LD is a valuable resource for researchers seeking to advance their understanding of liver diseases and develop innovative treatments.







