Partnerships
Empowering real-world evidence through unique strategic partnerships
Collaborate with Us to Advance
Real-World Evidence Solutions
At Target RWE, we understand the power of collaboration in driving innovation and excellence in real-world evidence (RWE) solutions. We are dedicated to forging strong partnerships with a diverse array of stakeholders, including disease experts, industry partners, healthcare institutions, and academic organizations, to develop the most comprehensive patient registries and disease-relevant datasets across key therapeutic areas.
By working together with leading professionals and organizations, Target RWE ensures that our data is robust, reliable, and reflective of real-world patient experiences.

Expert Key Opinion Leadership in Target RWE Registries
Each Target RWE registry is developed with multiple committees comprised of industry leaders to help determine the focus of each study design and help develop analyses to support publication efforts.

Academic Steering Committee
Each registry is led by a network of external key opinion leaders (KOLs) in our disease-area-focused academic steering committees representing the highest levels of thought leadership and providing input into registries, protocols, and study design.

Advisory Committee
Our unique model leverages the collaboration of scientific, clinical, and epidemiologic expertise to unlock breakthrough insights. Each advisory committee of industry partners, regulatory, and key opinion leaders helps guide research and address the challenges of each disease area.

Publications Committee
Our disease expert KOLs are additionally involved through our publications committees dedicated to enriching the landscape of published real-world evidence that helps improve patient outcomes.
Highly Engaged Healthcare Institutions Across
the United States
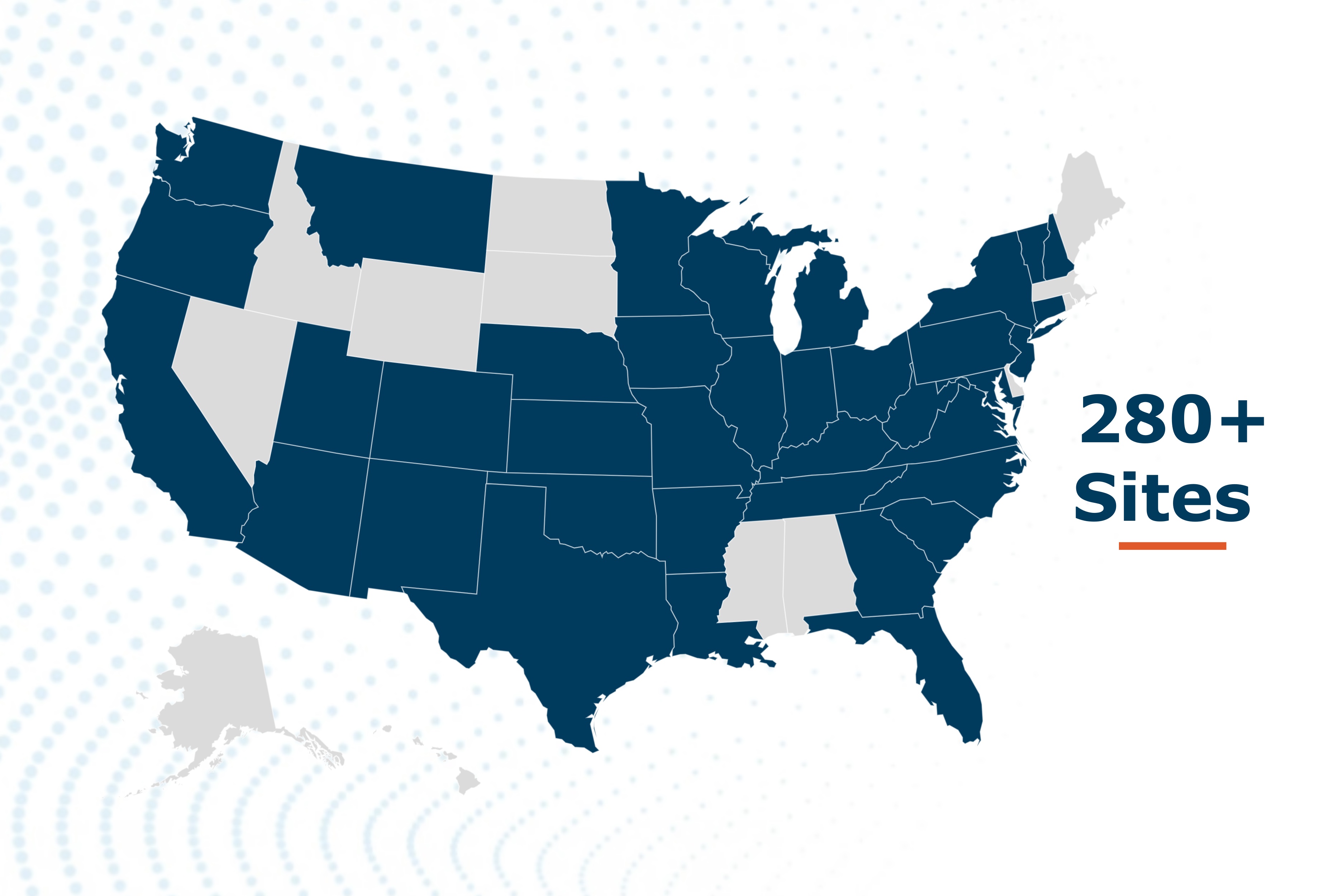
Target RWE deploys a unique site engagement model with ICD-10 screening resulting in large patient numbers enrolled in registries and the ability to tap into full EMR records for specific patient phenotypes for deep data collection. We have a network of 280+ highly engaged healthcare institutions across the U.S. that partner with us as study sites for our registries and help ensure patient engagement for active registry participation and data quality.
Delivering reliable and comprehensive real-world datasets with over 98% data completeness, our collaborative approach ensures that participating sites receive substantial benefits including:
-
Access to data
-
Financial compensation
-
Opportunity to select patients for Engaged studies
-
Rapid startup times for contracted sites - allowing for efficient project initiation
Join Our Network of Sites
Our partnerships with academic and community sites provides continuous collaboration to support any additional data needs required for partner use cases, offering flexibility in data collection.
Target RWE’s distinct model offers sites the chance to contribute to accelerating the generation of real-world evidence and improvement of patient care outcomes.
Academic Alliances Driving Innovation in Real-World Evidence Research
The American Association
for the Study of Liver Diseases (AASLD)
Target RWE has a strategic partnership with AASLD to support its Cirrhosis Quality Collaborative (CQC) by combining the CQC network with our TARGET-Liver Disease (LD) registry and analytics to advance research and improve the quality of care in patients with liver disease.
The American Gastroenterological
Association (AGA)
Our partnership with the AGA significantly expands real-world data available for serious, and often chronic, gastroenterological diseases. Target RWE has incorporated AGA’s eosinophilic esophagitis (EoE) severity index tool, I-SEE, into the TARGET-GASTRO registry.
Want to Partner with Target RWE?
By Submitting this form, you consent to your information used by Target RWE for the purpose of contacting you to better understand your potential interest in Target RWE and/or its services. For more information, see our privacy policy.
